Understanding Air Separation
Atmospheric air is a critical industrial resource, composed mainly of nitrogen, oxygen, and argon, with trace gases including carbon dioxide, neon, and helium. These gases have distinct physical properties, especially their boiling points. The boiling point variations form the basis of cryogenic air separation. Cryogenic distillation stands as the go-to way in industry for pulling these parts apart. It depends on managed temperature and pressure to turn air into liquid and divide it into separate components. Large-scale air separation equipment gets the job done well by using those boiling point differences in air parts.

Why Cryogenic Distillation Matters
Cryogenic distillation proves essential for making high-purity gases for industrial use. Oxygen holds an essential role in steel production. Nitrogen finds wide use in keeping things inert for electronics and metal work. Argon plays a key part in welding tasks and chip making. DINAK's cryogenic air separation systems aim to enhance gas purity while improving energy efficiency. Large-scale air separation equipment achieves efficient separation based on the differences in boiling points of various components in air.
Composition of Air and Boiling Points
Major Components of Atmospheric Air
- Nitrogen: 78%
- Oxygen: 21%
- Argon: ~1%
Other gases like carbon dioxide, neon, and helium exist in trace amounts.
Boiling Points of Key Gases
The success of cryogenic distillation comes from the clear differences in boiling points among air parts:
- Nitrogen: -196°C
- Argon: -186°C
- Oxygen: -183°C
Small-scale air separation equipment achieves efficient separation based on the differences in boiling points of various components in the air.
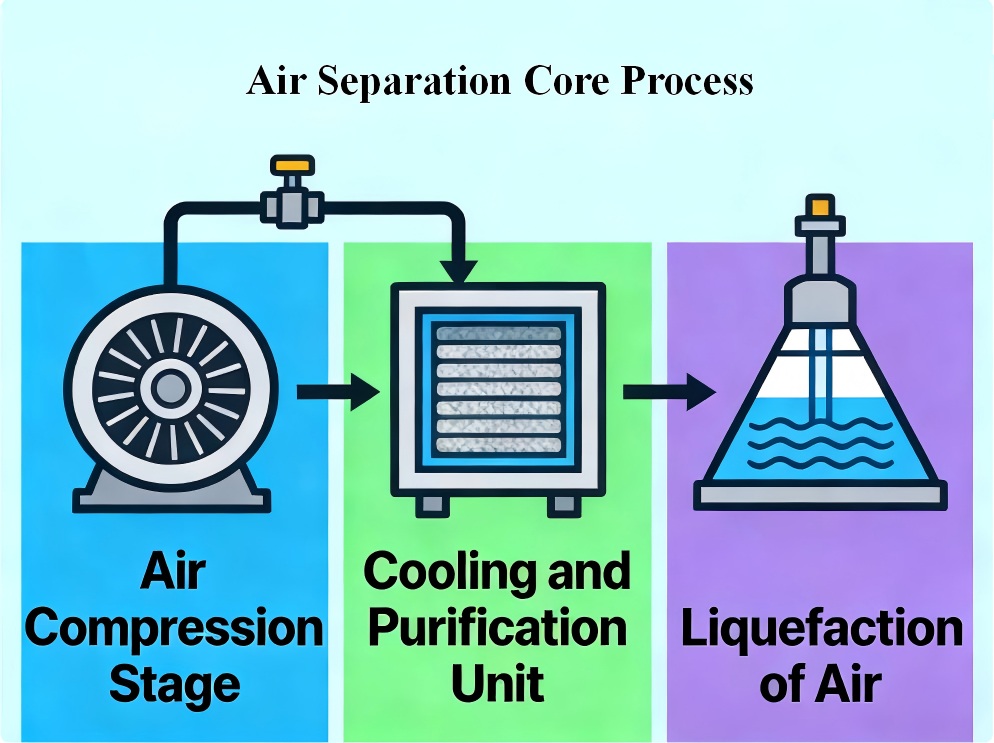
Air Separation Core Process Overview
Compression Stage
The process begins with ambient air being compressed by compressors. After compression in a high-efficiency air compressor, the raw air moves into the room-temperature molecular sieve adsorption purification system. There, it gets a full clean from things like water, carbon dioxide, and hydrocarbons. This step prepares the air for subsequent processing by increasing its pressure and temperature.
Cooling and Purification Stage
Next, the squeezed air cools down in the main heat exchanger. It gives off heat to the cold gases coming back. This early cooling helps set the stage for turning gas into liquid. At the same time, water vapor, CO₂, and hydrocarbons get pulled out via molecular sieve adsorption. That stops them from freezing in the cold work. The cleaned air swaps heat with the returning cold gas in the main heat exchanger. Then, after initial cooling, it heads to the fractionation tower system.
Liquefaction of Air
More cooling follows until the air drops below -180°C. At that point, it turns to liquid. Clean air swaps heat with the low-temperature product gas returning in the main heat exchanger. It cools to a very low point. The main cold maker, the turbo expander, keeps supplying the needed chill to the setup. It does this by doing work, and in the end, it turns the air into a liquid.
Fractional Distillation in the Column System
Structure of the Distillation Column
DINAK utilizes a two-column distillation system, consisting of a high-pressure (HP) column and a low-pressure (LP) column arranged sequentially. These columns have trays or packing to improve mixing between liquid and vapor. That aids better transfer of mass. In distillation separation, air cooled to its liquid point goes into a two-stage distillation column. There, nitrogen, oxygen, and argon get split one by one under tight control of temperature and pressure.
High-Pressure Column Functionality
Liquid air enters the HP column for the initial split. Lighter stuff like nitrogen turns to vapor first and climbs to the top. Meanwhile, heavier oxygen-rich liquid sinks down.
Low-Pressure Column Functionality
The liquid from the HP column bottom flows to the LP column for closer separation. The LP column runs at less pressure, which sharpens the split:
- High-purity nitrogen exits from the top.
- High-purity oxygen collects at the bottom.
Through several steps of mass and heat transfer inside the distillation column, high-purity nitrogen comes out at the top of the column. High-purity oxygen gathers at the bottom.
Role of Argon Separation Section
Argon's boiling point of -186°C lets workers pull it out between nitrogen and oxygen areas. A dedicated argon separation column employs a no-hydrogen full-distillation technique, which enhances safety, reduces energy consumption, and produces high-purity argon efficiently.
Product Collection and Storage
Oxygen Extraction
Oxygen reaching over 99% purity gathers at the LP column base. It can stay as a liquid in cryogenic tanks or turn to gas for pipe delivery. DINAK's setups often add a liquid oxygen pump for straight high-pressure output. Oxygen can be internally compressed via a liquid oxygen pump to provide high-pressure gas output safely and reliably.
Nitrogen Recovery
Nitrogen pulled from the LP column top can come as gas or turn to liquid with extra cooling help for longer storage.
Handling Argon
Argon comes out from its special spot between the HP and LP columns. Based on what users need, it might get more cleaning for top purity levels.
Process Control and Safety
Automation in Modern Systems
DINAK builds in smart PLCs and SCADA setups for close watch and handling of the process. DINAK's advanced instrumentation and control system enables real-time monitoring of key parameters, automated alarms, and protective functions. This keeps things like pressure, temperature, flow amounts, and gas cleanness in good ranges.
Safety Measures
DINAK's systems come with full safety tools:
- Overpressure relief valves.
- Oxygen concentration sensors.
- Emergency shutdown protocols.
- Regular preventive maintenance schedules.
DINAK provides peace of mind customer service as well as comprehensive long-term maintenance agreements, including all necessary spare parts, remote service, and minor or major repairs.
Conclusion
Cryogenic distillation keeps ranking as a top way to pull high-purity industrial gases from regular air. As a firm with more than 20 years in building full gas separation systems—from Small Scale ASU to Large-Scale ASU—we at DINAK keep pushing forward on cryogenic tech. We focus on better energy use, steady running, and lasting value for clients around the globe. With a broad and special background, we team up tightly with customers worldwide. We build answers fit to their wants. These steps raise output, cut waste, and stretch the life of the whole plant setup.
FAQ
Q: What is cryogenic distillation used for?
A: Cryogenic distillation is used to separate atmospheric air into components like oxygen, nitrogen, and argon based on their distinct boiling points under extremely low temperatures.
Q: How does an air separation plant work?
A: It compresses ambient air, removes impurities such as moisture and CO₂ through purification systems, cools it below -180°C until liquefied, then separates each gas via fractional distillation columns.
Q: Why are two columns used in cryogenic distillation?
A: The two-column system—high-pressure (HP) and low-pressure (LP)—enhances separation efficiency by leveraging different pressures for step-by-step refinement of gas purity.
Q: Is cryogenic air separation energy-intensive?
A: Yes; however, DINAK's systems utilize advanced turboexpanders and heat exchange optimizations that significantly reduce energy consumption compared to traditional methods.


-430x247.jpg)


内页小-430x246.jpg)

